- Biography
- Works
- Contact
The information below refers to the time of the award.
The biochemist Stephen C. West is a British citizen. He is a Senior Group Leader with Cancer Research UK’s London Research Institute, at Clare Hall Laboratories in South Mimms, where he directs a research group specialised in the study of DNA repair mechanisms. A Fellow of the Royal Society and of the Academy of Medical Sciences and a member of the European Molecular Biology Organisation, he was also awarded the Swiss Bridge Prize Award for Cancer Research in 2002.
Studies on DNA repair and therapeutic prospects
Stephen C. West has devoted most of his career to the study of enzymes involved in the repair of broken chromosomes. During the 1990s, scientists identified two genes, BRCA1 and BRCA2, defects in which predispose women to develop breast or ovarian cancer. Stephen C. West carried out pioneering work establishing the link between the action of these genes and DNA repair mechanisms, the processes which make it possible to repair our genome when it is damaged by X-rays, solar UV or certain chemical products.
Stephen C. West demonstrated that the protein BRCA2, the “product” of the gene of the same name, controls another genome-guarding protein, RAD51, and helps it to carry out its work of “mending” broken strands of DNA by telling it where and when to intervene. His work showed that, when BRCA2 is defective, repair cannot be carried out correctly.
These studies should have interesting therapeutic consequences, as a better knowledge of DNA repair mechanisms may make intervention possible. If we were able to block these repair processes in cancer cells, it should be possible to make tumours more sensitive to radiotherapy and to new chemotherapeutic treatments.
Stephen C. West has also studied the link between DNA repair systems and other hereditary diseases, in particular Ataxia with Oculomotor Apraxia, a neurological disease seen from childhood and characterised by an unstable gait and difficulty in moving the eyes. With the award, he also intends to study Fanconi’s anaemia, another rare hereditary disease associated with a predisposition to cancers and genetic instability.
Genome repair and hereditary breast cancer
DNA repair
Our genome, the famous DNA coiled up inside each of our cells, is relatively fragile. When exposed to X-rays, ionising radiation, solar UV, or certain chemical products, such as those found in cigarette smoke, the two strands of this long helical molecule can be broken or damaged.
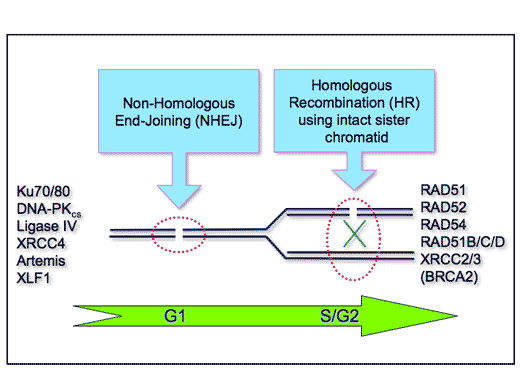
Fortunately, cells have several effective systems that can repair lesions in DNA (see the figure). One of these, used by simple organisms, such as yeasts and bacteria, is “homologous recombination”, in which undamaged DNA on the same arm of the sister chromatid acts as a template for the repair and restoration of its damaged homologue. Human cells also use this mechanism, but, in addition, can use another pathway, known as “non-homologous end-joining”, which brings together the two broken ends and “glues” them back together. However, when these processes are defective, the cells are not able to repair the breaks and die or become cancerous.
Stephen West specialises in the study of these repair mechanisms. Working first on simple organisms, such as bacteria, he characterised the enzymes involved in homologous recombination. He was then able to reproduce the main steps of this process in vitro, i.e. in the test tube.
Stephen West then turned to higher organisms. His aim was to understand DNA repair processes in human cells to find out how defective repair processes could lead to the appearance or development of diseases such as cancers or certain neurological diseases. In this context, he was particularly interested in certain hereditary forms of breast cancer.
Genes and hereditary cancers
During the mid-90s, scientists identified two genes that are involved in the development of hereditary breast cancers. When faulty, these genes, BRCA1 and BRCA2, significantly increase a woman’s risk of developing this type of cancer and are thought to be responsible for around 5% of all breast cancer cases. They have also been implicated in an increased risk of ovarian cancer.
It was known that, when BRCA1 and BRCA2 were defective, DNA was more sensitive to external agents liable to damage it and became more unstable. This led Stephen WEST to determine their exact role in genome repair.
He discovered that the protein BRCA2 (encoded by the gene of the same name) interacts with a key guardian of the genome, the protein RAD51, which forms a filamentous structure around the DNA and plays a critical role in the repair of damaged DNA. “Our findings suggested, that in the normal cell, BRCA2 protein controls when and where RAD51 does its repair work. It does this by binding to RAD51, keeping it in a state of readiness until DNA needs to be repaired. Then, in response to DNA damage, the two proteins relocate to the damaged site and initiate the repair of chromosomal breaks”. It is therefore easy to understand that, when the gene BRCA2, and thus the corresponding protein, is defective, RAD51 cannot carry out its job properly. This leads to chromosomal instability and opens the way to uncontrolled cellular proliferation and the development of cancers.
Potential therapeutic applications
These studies are of fundamental interest, as they allow a better understanding of the mechanisms by which genetic predispositions lead to the appearance of many hereditary breast cancers.
However, they could also have many interesting therapeutic consequences, as the better we understand the mechanisms of DNA repair, the more likely we are to be able to influence them. In fact, although these repair processes are essential for the correct functioning of normal cells, they can be an obstacle when we want to use radiotherapy or chemotherapy to destroy malignant cells. “If ways can be found to block these repair pathways in tumour cells”, says Stephen WEST, “it should be possible to make tumours more vulnerable to treatment with X-rays or chemotherapeutic agents”.
As regards the link between the DNA repair systems and other diseases, Stephen WEST showed that Ataxia with Oculomotor Apraxia, a hereditary neurological disease, is due to a defect in aprataxin, a protein which, like RAD51, plays an important role in protecting the genome against damage.
|
The pathways of DNA repair In vertebrates, breaks in the double-strand of DNA are repaired by two main mechanisms:
|
Dr Stephen C. WEST
Cancer Research UK
London Research Institute
Clare Hall Laboratories
UK – South Mimms, Herts EN6 3LD
T +44 1707-625868
F +44 1707-625811
