- Biography
- Works
- Contact
The information below refers to the time of the award.
A British citizen, Fiona POWRIE was born in 1963 in Luton (United Kingdom). She studied biochemistry at the University of Bath before undertaking a PhD in immunology in Don Mason’s Laboratory in Oxford. Following postdoctoral studies in the United States with Dr. Robert Coffman, she returned to Oxford in 1996 to establish her own laboratory as a Wellcome Trust Senior Research Fellow. In 2009, she was appointed as the inaugural Sidney Truelove Professor of Gastroenterology at the University of Oxford. She is also Head of the Experimental Medicine Division of the Nuffield Department of Medicine at the John Radcliffe Hospital, University of Oxford.
Fiona POWRIE is a Fellow of the Royal Society since 2011. She has already received numerous distinctions, notably in 2009 the Ita Askonas Prize, from the European Federation of Immunological Societies (EFIS) and the European Journal of Immunology (EJI), which is awarded to leading female immunologists.
The immune system and the intestinal flora
In the view of Fiona POWRIE, the digestive tract is really the “Wild West” for the immune system, which has to combat microbial pathogens while at the same time protecting the beneficial bacteria living in our intestines. A delicate balance exists between the system of defence and the intestinal flora which, if upset, results in intestinal inflammatory diseases.
Fiona POWRIE identified certain regulatory T cells (Treg) that police the immune response in the intestine, thus preventing our defence system from attacking bacteria that are of benefit to us. Furthermore she demonstrated that Treg cell deficiencies can lead to chronic intestinal inflammatory disease. She also showed that in the intestine a delicate equilibrium exists between two types of T cells, one of which causes inflammation while the other can counter the process. Her work is opening up new perspectives for the treatment of chronic intestinal inflammatory diseases.
The intestines are the Wild West of the immune system
The gastrointestinal tract is home to a large number and vast array of bacteria. In adults, the stomach and intestines contain trillions of bacteria – some ten times more than the total number of our own cells – grouped into about a thousand different species. This commensal flora is vital for the proper functioning of the organism, as it breaks down the numerous substances we have ingested.
For the immune system these bacteria are really like the “Wild West”, as Fiona Powrie says. The immune system has to combat microbial pathogens, while at the same time protecting the beneficial bacteria of the commensal flora. A delicate balance exists between immune defence and the intestinal flora which, if upset, results in inflammatory bowel diseases (IBD) such as Crohn’s Disease (CD) and Ulcerative Colitis (UC).
Regulatory T cells reinforce intestinal equilibrium
Fiona Powrie and her colleagues used mouse models of chronic intestinal inflammation in order to study how IBD can develop. This research showed that in the intestine a delicate equilibrium exists between two types of T cells: effector T cells which induce an inflammatory response, and regulatory T cells (Treg) which can counter this process.
Treg cells are a specialised population of T cells that police the immune system preventing it responding in a damaging way to our own tissues and to innocuous environmental antigens that we encounter at body surfaces. Treg cells require the transcription factor FOXP3 for development and in order to function, and humans who have mutations in FOXP3 develop a severe and potentially fatal inflammatory syndrome.
Treg cells utilise multiple mechanisms to control the immune response. This is in part dictated by the type of inflammatory response they are controlling. Treg cells are abundant in the intestine and produce high levels of the immune regulatory cytokine (interleukin IL-10) which is required for them to function in this site. Recent studies in humans have shown that loss of function mutations in a component of the receptor for IL-10 (IL-10RA) results in an aggressive IBD in children.
Given the immunosuppressive properties of Treg cells, there is a lot of interest in stimulating this pathway as a therapy in chronic inflammatory disease. Again the intestine is providing clues for how to do this. Work from Fiona Powrie’s lab and others has identified the intestine as a preferential site for the development of Treg cells. Specialised dendritic cells in the intestine can induce antigen-specific Treg cells. Recent studies suggest that intestinal bacteria themselves can induce Treg cells and promote IL-10 production. Indeed it seems likely that certain intestinal bacteria specifically promote the Treg/IL-10 axis to allow a peaceful coexistence within the host.
Intestinal tissue cells including epithelial cells (which cover the internal surface of the digestive tract), and stromal cells (connective tissue cells) as well as immune cells (such as macrophages and dendritic cells) express pattern recognition receptors that allow them to sense and respond to bacteria, thus facilitating pathways of mucosal host defence and immune regulation. In intestinal inflammatory diseases this normally beneficial activity of our commensal bacteria is disrupted, and these bacteria become the target of attack by the immune system.
The pivotal role of the IL-23/Type 17 cytokine response in intestinal inflammation
The cytokine IL-23 plays a pivotal role in orchestrating intestinal inflammation. Fiona Powrie’s team has shown that IL-23 acts directly on T cells to promote pathological Th17 type responses at the expense of immune suppressive regulatory T cells. In addition, IL-23 drives a novel population of innate lymphoid cells (ILC) that mediate colitis through the production of Th17 associated cytokines. Like Th17 cells, IL-23-driven ILC are dependent on the transcription factor ROR t, indicating striking functional parallels between innate and adaptive lymphoid populations in the intestine. Together these results highlight the multiple activities of IL-23 that mediate tissue inflammatory responses. Further understanding of the molecular signatures associated with IL-23R signalling in T cells may provide novel therapies for intestinal inflammatory disease.
t, indicating striking functional parallels between innate and adaptive lymphoid populations in the intestine. Together these results highlight the multiple activities of IL-23 that mediate tissue inflammatory responses. Further understanding of the molecular signatures associated with IL-23R signalling in T cells may provide novel therapies for intestinal inflammatory disease.
Faecal microorganisms
In addition to changes in the intestinal immune system it is also clear that the composition of faecal microorganisms undergoes marked changes during IBD. Recent evidence suggests that certain bacterial species shape the immune response in different directions. For example, a member of the group of the gram-positive Clostridium cluster has been shown to induce Th17 accumulation, while other members of this group induce IL-10 expression promoting an immune regulatory response.
Fiona Powrie and her colleagues will continue to investigate host microbial interactions in the intestine. In particular to establish how commensal bacteria can promote immune system tolerance and how we may harness this for enhanced therapeutics in chronic inflammatory diseases of the intestine.
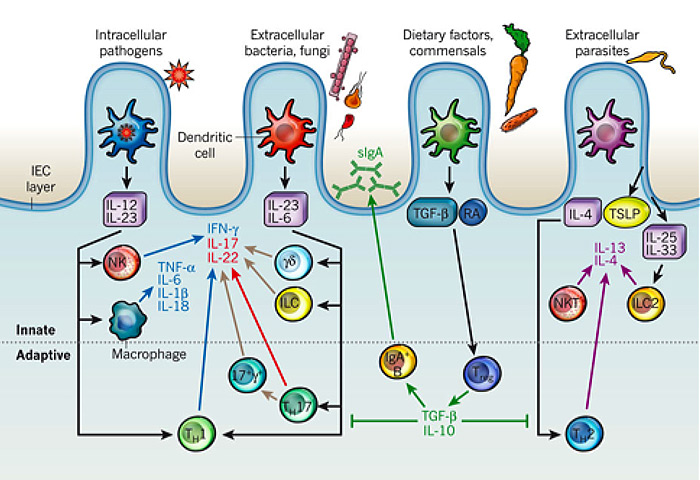
Fig. 1
Immune effector modules that drive intestinal inflammation are conserved across innate and adaptive leukocytes and can be controlled by regulatory T cells. (Adapted from an article by Maloy K.J. and Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Published in Nature (2011) 474: 298-306, with permission).
Fiona Powrie FRS
Sidney Truelove Professor of Gastroenterology
Translational Gastroenterology Unit
Experimental Medicine Division
Nuffield Department of Medicine
John Radcliffe Hospital
Headley Way, Headington
Oxford OX3 9DU (UK)
Tél.: +44 (0)1865 220 137 (PA) +44 (0)1865 222 909 (direct)
fiona.powrie@path.ox.ac.uk
http://www.ndm.ox.ac.uk/principal-investigators/researcher/fiona-powrie
http://www.expmedndm.ox.ac.uk/powrie-mucosal-immunology-group
