- Biography
- Works
- Contact
The information below refers to the time of the award.
Peter Hegemann was born 1954 in Munster, studied chemistry in his home town and then in Munich, where he was awarded his PhD in biochemistry. He then left for the USA and post-doctoral work at the University of Syracuse (State of New York). On his return to Germany in 1986, he was a research group leader at the Max-Planck Institute for Biochemistry, after which he was named Professor of Biochemistry at the University of Regensburg. Since 2004, he holds the position of Professor of Experimental Biophysics at the Humboldt University, Berlin. Peter Hegemann is a member of the German National Academy of Sciences, Leopoldina.
Georg Nagel was born 1953 in Weingarten near Ravensburg. He studied biology and biophysics at the recently created University of Konstanz. After teaching for several years at high school in Switzerland, he continued his education and was awarded his PhD in biology and biophysics at the University of Frankfurt am Main in 1988. Thereafter he left for the USA for post-doctoral training at Yale University, then Rockefeller University. He returned to Germany in 1992, where he became a group leader in the Department of Biophysical Chemistry at the Max-Planck Institute of Biophysics. Since 2004, he is Professor of Molecular Plant Physiology and Biophysics at the University of Wurzburg (Bavaria).
Peter Hegemann and Georg Nagel have already shared several distinctions, notably the Wiley Prize in Biomedical Sciences, USA (2010), and the Karl-Heinz-Beckurts Prize (2010) and Klaus-Joachim-Zülch Prize (2012) in Germany.
From green alga to neurosciences
It all started back in the 1980’s, when Peter Hegemann tried to understand how a microscopic green alga, Chlamydomonas reinhardtii, achieves to move towards or away from a light source. After about 10 years of research, he suggested that a closely linked protein complex consisting of rhodopsin and a calcium channel depolarises the alga’s membrane which is sensed by the flagella that modify the movement according to light intensity and color. Peter Hegemann identified the rhodopsin genes in a Japanese cDNA bank and sent the cDNA to Georg Nagel. Georg Nagel succeeded in expressing the rhodopsin proteins in animal cells and in characterizing in detail their function. He confirmed and extended Peter Hegemann’s hypothesis by demonstrating that the rhodopsins – which he called Channelrhodpsins – function as light-driven ion channels.
As the two biochemists had suggested, this mechanism does not only function in algae. The Channelrhodopsins can be expressed, for example, in nerve cells (neurons) of numerous animal species, from worms to primates, to make them light-sensitive and to study the function of selected neurons in the context of their network.
The two German scientists thus ushered in a new discipline – optogenetics – chosen by the Journal Nature Methods as the “Method of the Year” for 2010. It has indeed emerged that light can stimulate neurons in higher species, opening the way for numerous medical applications. It is to be hoped that light may be used to give back rudimentary vision to blind people, to stimulate the deep brain of patients suffering from Parkinson’s, even to influence cardiac rhythm for the treatment of heart failure.
A green alga that moves under the influence of light
For many people, the word “algae” means nothing more than “pond scum”, and indeed this was the expression used by the Journal Nature Methods editorialist in 2010 when praising the scientific work of Peter Hegemann and Georg Nagel.
The “pond scum” we are talking about is actually called Chlamydomonas reinhardtii. This microscopic green alga is much in demand among researchers who use it for studying fundamental biological processes such as photosynthesis, cell division, and flagella motion or how organisms move.
Another distinctive characteristic of this type of algae is that its movements are controlled by light. Back in the 1980’s Peter Hegemann turned his attention to this phenomenon and began examining the mechanisms that make Chlamydomonas photosensitive.
One of the members of his team was the first to study the electrophysiology of this alga. Following stimulation by using luminous flashes, he measured the electric current generated by its photoreceptor and the current subsequently appearing in the alga’s flagella. He thus observed that electric currents generated by light (photocurrents) depend on the intensity of the light and that they affect the movements of the flagella and thus the position of the alga with respect to the light source.
Given the considerable speed with which the photocurrent was generated, Peter Hegemann and his colleagues posed the hypothesis that the algal photoreceptor was made up of a rhodopsin associated with an ion channel, together forming a single protein complex.
Microbial rhodopsins
Rhodopsins are light-sensitive proteins which were first found in the retinas of animals’ eyes. They consist of opsin (a protein) and of retinal (vitamin A). Rhodopsins modulate indirectly ion channels that transport sodium/ calcium/ potassium ions across the membrane. Astonishingly, rhodopsins have also been found in microbes (archaebacteria) which survive extreme environmental conditions by energy capture from bright sunlight, using “microbial type” rhodopsins.
Georg Nagel was the first to bring (via gene transfer) microbial rhodopsins into animal cells and to show that they are completely functional in this unusual environment. He demonstrated so far unknown properties of microbial rhodopsins by studying their working in animal cells with electrophysiological methods.
For years, Peter Hegemann and his team tried to clone the photoreceptor ofChlamydomonas, but they did not succeed due to the instability and heterogeneous characteristics of the proteins involved.
The breakthrough occurred in 2001, when one of Peter Hegemann’s colleagues identified a new sequence, resembling a microbial rhodopsin, in the Chlamydomonas cDNA bank of a Japanese research center. It was at this point that Peter Hegemann started working with Georg Nagel, by sending him two of these DNA sequences.
Georg Nagel was already an established electro-physiologist who was working on ion (salts) transport via cellular membranes. He had notably studied the regulation processes of a human membrane protein named CFTR (Cystic Fibrosis Transmembrane conductance Regulator), which is defective among patients suffering from Cystic Fibrosis. As mentioned, he also pioneered the expression of microbial rhodopsin in animal cells, particularly in the oocytes (immature female sexual cells) of Xenopus laevis – aquatic toads from southern Africa.
Channelrhodopsins
The approaches adopted by Peter Hegemann and Georg Nagel were thus complementary, and their collaboration for the study of the photoreceptors of theChlamydomonas reinhardtii green alga proved to be fruitful.
Once he had received the DNA sequences of the rhodopsins from Peter Hegemann, Georg Nagel expressed the proteins in the Xenopus oocyte cells and studied their functioning. By analysing the photocurrents stimulated by light, he reached the conclusion that Chlamydomonas rhodopsins form “light-gated” ion channels, i.e. channels which only become active and allow the transport of ions through the membranes when in the presence of light.
He proposed to call these rather special ion channels “channelrhodopsins” (ChR), of which Georg Nagel and Peter Hegemann have found two kinds – ChR1 and ChR2.
The birth of optogenetics
The researchers also expressed ChR2 in human kidney cells (see Figures A and B), which led them to suggest that such an ion channel ought to function in organisms other than Chlamydomonas reinhardtii green alga. They were right.
Georg Nagel had been working with Alexander Gottschalk, a neurobiologist from the University at Frankfurt am Main who was using the small nematode C. elegans. The two scientists expressed an enhanced ChR2 mutant in this worm’s cells, and in 2005 demonstrated that worm behaviour could be modulated by light.
Since then, numerous other teams around the world have shown that the ion channel is expressed in mouse brain tissue, in the spinal chord of chicken embryos and in the retina of blind mice. Hence thanks to the pioneering work of Peter Hegemann and Georg Nagel, optogenetics was born. It was designated “Method of the Year 2010” by the JournalNature Methods.
This new discipline, which seeks to express photosensitive proteins in certain neural cell populations and to activate them using light stimuli, opens the way for numerous medical applications, especially in neurology. It is to be hoped that one day, light may be used to give back rudimentary vision to blind people, to stimulate the deep brain of patients suffering from Parkinson’s, even to influence cardiac rhythm for the treatment of heart failure.
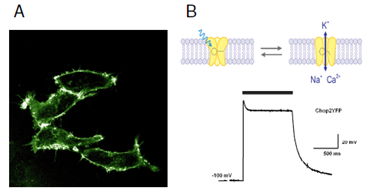
Fig. A
Channelrhodopsin-2 (ChR2), fused to a fluorescent protein (YFP), labels the plasma membrane of human embryonic kidney cells.
Fig. B
Light (indicated by grey bar) opens ChR2YFP cation channels, leading to a strong and reversible depolarisation.
Professeur Peter Hegemann
Experimental Biophysics
Humboldt-Universität zu Berlin
Invalidenstrasse 42
10115 Berlin (Allemagne)
Tél. +49 30 2093 8681 (direct)
Tél. +49 30 2093 8830 (assistante)
hegemann@rz.hu-berlin.de
http://www2.hu-berlin.de/biologie/expbp
Professeur Georg Nagel
Julius-von-Sachs-Institute
Biocenter – University of Würzburg
Julius-von-Sachs-Platz 2
97082 Würzburg (Allemagne)
Tél. +49 931 318 6143 (direct)
Tél. +49 931 318 9199 (assistante)
nagel@uni-wuerzburg.de
http://www.bot1.biozentrum.uni-wuerzburg.de/forschung/nagel/
